When a brand-name drug’s patent expires, generics should step in-cheaper, faster, and just as effective. But in reality, many patients wait years longer than they should. Why? Because patent litigation has become a tool to block competition, not just protect innovation. In the U.S., generic drug entry now takes an average of 28 months after brand approval-double what it was in 2005. For cancer drugs, delays stretch to over five years past patent expiration. This isn’t accidental. It’s systemic.
The Hatch-Waxman Act: A System Designed to Balance Competition and Innovation
The Hatch-Waxman Act of 1984 was meant to create a fair middle ground. It gave brand-name companies extra patent time to make up for delays in FDA approval. At the same time, it gave generic manufacturers a clear path to enter the market by filing an Abbreviated New Drug Application (ANDA). The key? The Paragraph IV certification. When a generic company says, “Your patent is invalid or we don’t infringe it,” they’re legally challenging the brand’s monopoly. That triggers a 30-month clock. During that time, the FDA can’t approve the generic-unless the court rules in the generic’s favor. This system was supposed to encourage early challenges. But over time, it became a bottleneck. Instead of one clean fight, brand companies now file multiple patents, sometimes years apart, to reset the clock again and again. This is called serial patent litigation. And it’s working.The Orange Book: Where Patents Are Listed-And Sometimes Abused
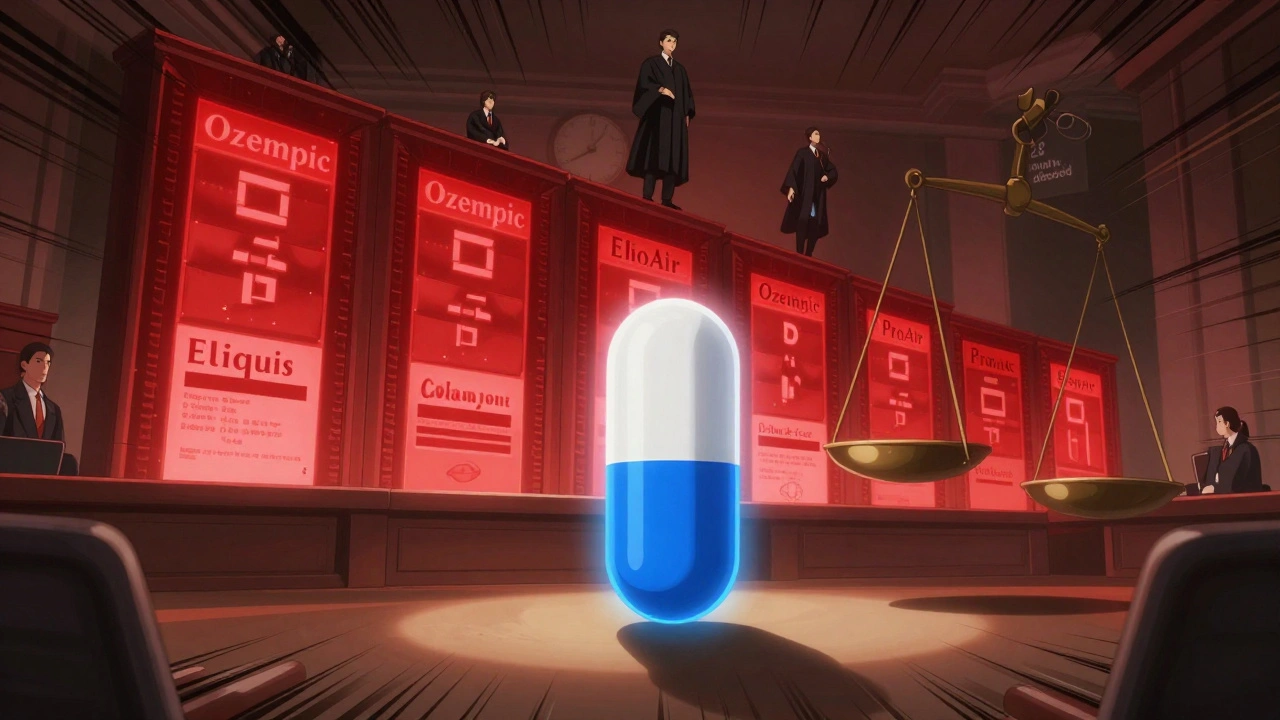
The FDA’s Orange Book is the official list of patents tied to brand-name drugs. Only certain patents belong there: those covering the active ingredient, formulation, or method of use. But companies have found loopholes. They list patents for things like inhaler devices, packaging, or manufacturing equipment-even when those aren’t part of the actual drug. In 2025, a landmark case involving Teva and Amneal over the asthma inhaler ProAir® HFA changed the game. Judge Chesler ruled that patents on the dose counter in the inhaler didn’t qualify for Orange Book listing because they didn’t claim “the drug for which the application was approved.” The drug was albuterol sulfate. The counter? Just a piece of hardware. That ruling could invalidate 15-20% of currently listed patents. The FDA is now proposing new rules requiring brand companies to certify under penalty of perjury that every listed patent meets the legal standard. That’s a big deal. Right now, there’s little consequence for listing junk patents. Under the new rules, false claims could mean fines or even criminal liability.Where Lawsuits Are Fought: The Eastern District of Texas
Not all courts are created equal. In 2024, 38% of all patent lawsuits were filed in the Eastern District of Texas. Why? Because it’s known for being fast, predictable, and favorable to patent holders. After the TC Heartland decision briefly shifted cases away, the district bounced back-thanks to experienced judges and local rules that favor plaintiffs. Compare that to the District of Delaware, which used to be the go-to for pharmaceutical cases. Now it’s third in filings. Generic companies hate this. They argue it’s forum shopping-brand companies choosing courts where they’re more likely to win, not where the case belongs. But until Congress or the Supreme Court steps in, the Eastern District remains the battlefield.
Pay-for-Delay: The Settlement That Isn’t a Settlement
Here’s the twist: sometimes, brand and generic companies don’t fight to the end. They settle. And in many cases, the brand pays the generic to stay off the market. That’s called a “pay-for-delay” agreement. The FTC calls it anticompetitive. The industry calls it smart business. The FTC has challenged over 300 improper Orange Book listings in 2024 alone. But here’s the contradiction: according to IQVIA’s 2025 report, patent settlements actually speed up generic entry-on average, by more than five years before patent expiration. How? Because without the chance to settle, generics might not file Paragraph IV challenges at all. Why risk a $10 million lawsuit if you can’t win? The fear of losing keeps many off the field. So the real problem isn’t just the settlements. It’s the system that forces them. If generics can’t settle, they file fewer applications. And fewer applications mean fewer generics on the market.Patent Thickets: When One Drug Has Over 150 Patents
Oncology drugs are the worst offenders. Semaglutide (Ozempic, Wegovy, Rybelsus) has 152 patents protecting it. Eliquis has 67. That’s not innovation-it’s a legal maze. Dr. Rachel Sachs of Washington University calls this a “patent thicket.” Each patent is weak on its own. But together, they create a wall. Generic companies can’t challenge them all at once. They pick one. Win it? The brand files another. Win that? Another pops up. The process drags on for years. By the time a generic finally wins, the original patent has expired-but the market has already moved on. Patients didn’t get cheaper drugs. The brand kept its profits. The I-MAK 2025 report found that the average number of patents per small molecule drug has jumped from 37 in 2010 to 78 today. For biologics? Even higher. This isn’t about protecting inventions. It’s about extending monopolies.

Regan Mears
December 10, 2025 AT 12:06This is heartbreaking. I’ve seen friends choose between insulin and rent-and it’s not a metaphor, it’s their reality. The system isn’t broken, it’s rigged. And the people who suffer? They don’t get a seat at the table. I just wish more people could see how many lives are hanging on this.
Ben Greening
December 11, 2025 AT 11:57The structural incentives within the Hatch-Waxman framework have been systematically distorted by litigation strategy rather than innovation. The aggregation of patent filings, particularly in oncology, represents a form of regulatory arbitrage that undermines the statutory intent of promoting generic competition.
Nikki Smellie
December 12, 2025 AT 18:47Have you ever wondered who really owns the FDA? 🤔 Big Pharma pays the regulators behind the scenes. The Orange Book? A joke. The 'new rules'? A distraction. They’re all in on it-doctors, lawyers, judges, even the FTC. It’s a pyramid scheme with pills instead of money. And we’re the bottom layer. 💔
Neelam Kumari
December 14, 2025 AT 09:25Oh please. You Americans think your patent system is the pinnacle of civilization? In India, generics are made in villages with no lawyers, no lawsuits, and people actually get medicine. You’ve turned healthcare into a courtroom sport. Pathetic.
Queenie Chan
December 14, 2025 AT 22:38Imagine a drug patent like a haunted house-every time you think you’ve cleared one room, another door slams open with a new ghost patent. 152 patents on Ozempic? That’s not innovation. That’s a legal haunted mansion built on the bones of patients who can’t afford their meds. And the worst part? The ghosts are all paper-thin. Just paperwork with teeth.
Stephanie Maillet
December 15, 2025 AT 16:16There’s a deeper question here, isn’t there? We say we value life-but we’ve built a system where profit is the only metric that matters. When a company can legally delay a life-saving drug for five years because it’s cheaper to litigate than to compete… what does that say about us? Not about the law. About our soul.
David Palmer
December 17, 2025 AT 04:02So let me get this straight-companies pay each other to NOT sell cheaper drugs? That’s not business. That’s just being lazy and greedy. Why not just open a lemonade stand and charge $500 a cup? Same logic.
Doris Lee
December 17, 2025 AT 13:04There’s hope. People are waking up. I’ve seen local pharmacies start talking to patients about generic alternatives-and they’re fighting back. Change starts small. Keep pushing. You’re not alone.
Michaux Hyatt
December 17, 2025 AT 17:58One thing people miss: IPRs were supposed to be the hero. But now the Supreme Court’s ruling makes it harder for generics to use them unless they’ve been sued first. That’s like forcing someone to get punched before they can defend themselves. It’s backwards.
Raj Rsvpraj
December 18, 2025 AT 23:25India? Please. Your generics are often substandard, untested, and exported to Africa under false labels. You don’t fix the problem-you export it. The U.S. system, flawed as it is, at least ensures quality. Your jealousy is showing, and it’s ugly.
Jack Appleby
December 19, 2025 AT 23:08Actually, the FTC’s 2024 count of improper Orange Book listings was 317-not ‘over 300’-and the 13.9B figure is derived from a 2023 RAND study, not a direct FTC calculation. Also, Semaglutide’s patent count is 147, not 152. Precision matters. You’re sloppy.
Frank Nouwens
December 21, 2025 AT 14:40It is worth noting that the Eastern District of Texas, while perceived as plaintiff-friendly, has demonstrated consistent procedural efficiency and judicial expertise in complex patent matters. The shift in forum preference may reflect logistical and strategic considerations rather than systemic bias.
Kaitlynn nail
December 23, 2025 AT 04:35Patent thickets = legal fog. Patients = lost in it. That’s the whole story.
Aileen Ferris
December 24, 2025 AT 06:50the whole thing is just a big scam. why do we even have patents if no one can afford the meds? also, i think the orange book should be called the orange lie book. lol