When you pick up a prescription at the pharmacy, the label on the bottle might look like any other. But if it’s a controlled substance, that label carries legal weight-and the numbers and symbols on it tell a story about risk, regulation, and safety. Understanding controlled substance labels and Schedule codes are federal classifications under the Controlled Substances Act (CSA) that rank drugs by abuse potential and medical value isn’t just for pharmacists or doctors. It’s for anyone who takes these medications-or knows someone who does.
What Are Schedule Codes, Really?
The U.S. government doesn’t treat all controlled drugs the same. Since 1970, the Controlled Substances Act (CSA) is the federal law that organizes drugs into five categories, or schedules, based on medical use, abuse potential, and safety has divided drugs into five schedules. These aren’t arbitrary. Each level reflects real data on how likely a drug is to cause dependence, how dangerous it is when misused, and whether doctors actually prescribe it.
Here’s how it breaks down:
- Schedule I: No accepted medical use in the U.S. High abuse potential. Examples: heroin, LSD, marijuana (still federally). These can’t be prescribed.
- Schedule II: High abuse potential, but accepted medical use. High risk of addiction. Examples: oxycodone, fentanyl, Adderall, morphine.
- Schedule III: Moderate to low abuse potential. Less risk than II. Examples: ketamine, hydrocodone/acetaminophen (Vicodin), anabolic steroids.
- Schedule IV: Low abuse potential. Examples: Xanax, Valium, Ambien, tramadol.
- Schedule V: Lowest abuse potential. Often available with minimal restrictions. Examples: cough syrups with small amounts of codeine, pregabalin (Lyrica).
Some drugs appear in multiple schedules depending on their form. Codeine, for example, is Schedule II when sold alone, Schedule III in combo pills like Vicodin, and Schedule V in low-dose cough syrups. That’s why the label matters more than the drug name.
What’s on the Label? Decoding the Fine Print
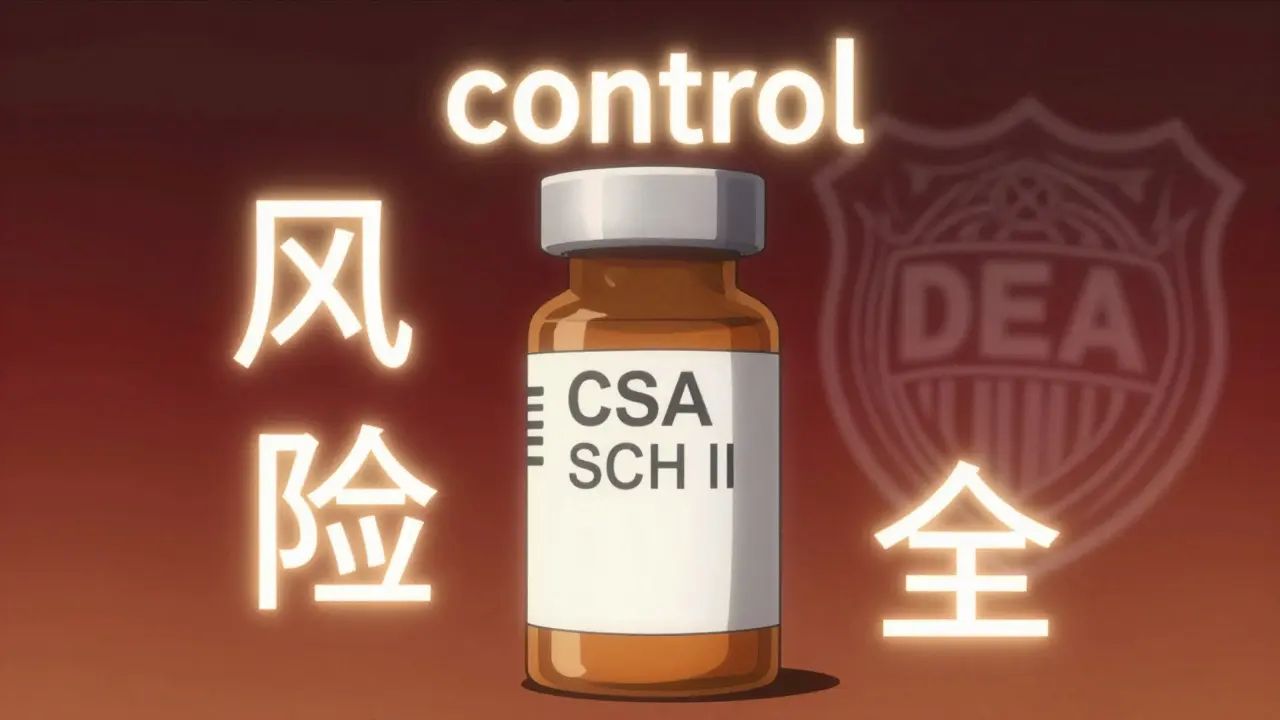
Look closely at the prescription bottle. You’ll see more than just your name and dosage. There’s a code-often labeled CSA SCH or CSN-that tells the pharmacist exactly how to handle the prescription.
For Schedule II drugs, the label will show CSA SCH II. That means:
- No refills allowed. Ever.
- Must be written on a physical, tamper-resistant prescription pad in most states.
- Electronic prescriptions are allowed in some states, but strict rules apply.
- Pharmacists must verify the DEA number of the prescriber.
Schedule III and IV drugs? They can be refilled up to five times in six months. Many are now prescribed electronically. You’ll see CSA SCH III or CSA SCH IV on the label. Schedule V? Sometimes you can buy them over the counter-like certain cough syrups-but the pharmacist still has to log the sale.
Here’s a quick reference:
| Schedule | Abuse Potential | Refills Allowed? | Prescription Format | Common Examples |
|---|---|---|---|---|
| Schedule I | High | None (not prescribable) | N/A | Heroin, LSD, marijuana (federally) |
| Schedule II | High | No | Physical or electronic (state-dependent) | Oxycodone, fentanyl, Adderall |
| Schedule III | Moderate to low | Up to 5 in 6 months | Electronic or physical | Vicodin, ketamine, anabolic steroids |
| Schedule IV | Low | Up to 5 in 6 months | Electronic or physical | Xanax, Valium, Ambien |
| Schedule V | Very low | Yes, often OTC with limits | Electronic, physical, or OTC | Cough syrup with codeine, pregabalin |
Why Does This System Exist?
The goal isn’t to restrict access-it’s to prevent misuse. The Drug Enforcement Administration (DEA) is the federal agency responsible for enforcing the Controlled Substances Act and tracking controlled substance distribution created this system to build a "closed loop"-tracking every pill from manufacturer to patient. Every pharmacy, doctor, and distributor must have a DEA registration number. That number starts with two letters (like "AB" or "MC") followed by seven digits. If a prescription doesn’t have a valid DEA number, the pharmacy can’t fill it.
It’s not perfect. A 2022 DEA audit found that 43% of compliance issues involved incomplete or missing records for Schedule II prescriptions. That’s a lot of paperwork. Pharmacists say it adds 15 minutes per prescription just to verify everything. But the system works. In 2022, a survey of 1,245 pharmacists showed that Schedule III and IV drugs made up over 92% of all controlled substance prescriptions filled. That means most people are getting lower-risk meds-exactly the system was designed to do.

Where the System Gets Messy
Here’s the big contradiction: marijuana. Federally, it’s Schedule I-no medical use, high abuse risk. But 38 states have legalized it for medical use. That creates confusion for patients, doctors, and pharmacies. A patient in Toronto might be prescribed medical cannabis legally under Canadian law, but if they’re traveling to the U.S., even a prescription from a U.S. doctor for marijuana is illegal under federal law.
Another issue: synthetic drugs. The DEA adds new substances to Schedule I every year-17 in 2022-2023 alone. These are lab-made chemicals, often sold as "bath salts" or "spice," and they’re dangerous. But the process to schedule them takes months. By the time the DEA acts, new versions are already on the street.
And then there’s the debate over whether the system reflects real risk. A 2023 survey by Deloitte found that 68% of healthcare experts expect at least two Schedule I drugs to be rescheduled by 2028. The most likely candidate? Marijuana. In August 2023, the Department of Health and Human Services recommended moving marijuana to Schedule III-meaning it could be prescribed legally under federal law. If that happens, it would be the biggest change since 1970.
What This Means for You
If you’re prescribed a Schedule II drug-like oxycodone for chronic pain-you’ll need a new prescription every time. No calling in for refills. You’ll get a physical script with a special barcode or security features. You might be asked to show ID. You might be limited to a 30-day supply. That’s not bureaucracy-it’s protection.
For Schedule IV drugs like Xanax, you might get a refill. But if you’re taking it long-term, your doctor will monitor you closely. Why? Because even low-risk drugs can become habit-forming. A 2023 Reddit thread with 342 pharmacists showed that 78% believe the current system creates unnecessary barriers, especially for Schedule II patients. But 82% also agreed that the structure helps them spot misuse early.
Bottom line: the label isn’t just a reminder of your dose. It’s a legal document. It tells the pharmacy how to store it, how to record it, and whether you can get it again next month. It’s why you can’t just walk into a pharmacy and buy fentanyl like you would ibuprofen.

What’s Changing? What to Watch For
The system is under pressure-and change is coming. The DEA’s 2023 Strategic Plan says they plan to cut the time it takes to schedule a new drug from two years to one. That’s huge. They’re also testing digital tracking systems to replace paper logs, which could reduce errors.
More importantly, the potential rescheduling of marijuana could reshape the entire framework. If marijuana moves to Schedule III, it would set a precedent. Other drugs-like MDMA for PTSD treatment or psilocybin for depression-could follow. Experts predict we’ll see a six- or seven-schedule system within 15 years to better separate risk levels.
For now, if you’re taking a controlled substance, pay attention to the label. Know your schedule. Ask your pharmacist if you’re unsure. And if you’re switching doctors or pharmacies, make sure your records are clear. A simple mistake-like a missing DEA number or an expired script-can mean waiting days for your medication.
Controlled substance labels aren’t meant to confuse. They’re meant to protect. Understanding them helps you take control-not just of your meds, but of your safety.
What does CSA SCH II mean on a prescription label?
CSA SCH II means the medication is classified under Schedule II of the Controlled Substances Act. This indicates a high potential for abuse and dependence, but it has accepted medical use. Prescriptions for Schedule II drugs cannot be refilled, must be written on tamper-resistant paper in most states, and require a valid DEA number from the prescriber.
Can I get a refill on a Schedule III prescription?
Yes. Schedule III prescriptions can be refilled up to five times within six months from the date the prescription was issued. These medications are considered to have a lower abuse potential than Schedule II drugs. Electronic prescriptions are allowed, and partial fills are permitted if the full amount isn’t needed.
Why is marijuana still Schedule I if it’s legal in my state?
Marijuana remains federally classified as Schedule I because the Controlled Substances Act is a federal law, and state laws don’t override it. Although 38 states have legalized medical marijuana, the federal government still considers it to have no accepted medical use and a high potential for abuse. However, in August 2023, the Department of Health and Human Services recommended rescheduling marijuana to Schedule III, which could change federal policy in the near future.
What’s the difference between Schedule II and Schedule IV drugs?
Schedule II drugs have a high potential for abuse and can lead to severe psychological or physical dependence. They include strong opioids like oxycodone and stimulants like Adderall. Schedule IV drugs have a lower abuse potential and include benzodiazepines like Xanax and sleep aids like Ambien. Schedule II prescriptions cannot be refilled; Schedule IV prescriptions can be refilled up to five times in six months.
Can I buy Schedule V medications without a prescription?
Some Schedule V medications, like certain cough syrups containing small amounts of codeine or antidiarrheal medicines with diphenoxylate, can be purchased without a prescription-but only under the supervision of a pharmacist. The pharmacist must record the sale, limit the quantity, and verify your identity. These drugs have the lowest abuse potential of all controlled substances.
How do I know if my medication is a controlled substance?
Check the prescription label. It will clearly state "CSA SCH" followed by a number (II, III, IV, or V). If you’re unsure, ask your pharmacist. Your doctor’s prescription will also indicate whether it’s a controlled substance. Common controlled substances include opioids, stimulants, sedatives, and certain anti-anxiety medications.
What happens if a pharmacy fills a Schedule II prescription without a valid DEA number?
The pharmacy could face serious penalties, including fines, loss of DEA registration, or even criminal charges. DEA regulations require that every controlled substance prescription include a valid DEA number from the prescriber. Pharmacies are required to verify this number before dispensing. If the number is missing, fake, or invalid, the prescription cannot be filled.
Next Steps: What to Do Now
If you’re taking a controlled substance:
- Always check the label for the CSA schedule code.
- Keep track of refill limits-especially for Schedule II drugs.
- Store your medications securely. Schedule II drugs are often targeted for theft.
- Ask your pharmacist if you’re unsure about your prescription’s status.
- Never share your controlled medication-even if it’s for pain or anxiety. It’s illegal and dangerous.
If you’re a caregiver or family member, learn the schedule of the medications your loved one takes. Understanding the risks helps you spot signs of misuse early.
The system isn’t flawless. But it’s the best tool we have to balance access with safety. Knowing what the labels mean gives you power-not just over your meds, but over your health.

Andrew Freeman
January 16, 2026 AT 02:59csa sch ii? more like csa sch why do i have to wait 3 days for my pain meds again
Sarah -Jane Vincent
January 16, 2026 AT 21:22they say schedule ii is high risk but let me guess the same people who scream about opioids are the ones who think weed is just a plant and not a gateway to federal prison. wake up. the system is rigged to keep the rich on xanax and the poor on street fentanyl.
Dylan Livingston
January 18, 2026 AT 00:07Oh sweet heavens. Another post that treats the DEA like it’s the benevolent guardian of human dignity. Let’s not forget that the entire schedule system was born out of 1970s moral panic, racial targeting, and the fact that Nixon wanted to criminalize hippies and Black communities under the guise of "public health." And now we’re all supposed to be grateful for the paperwork? The fact that you can’t refill a prescription for oxycodone but your neighbor can buy 200 pills of Adderall online from a shady pharmacy in Cambodia speaks volumes about how rational this whole circus is.
Anna Hunger
January 19, 2026 AT 04:06It is imperative that patients remain vigilant in understanding the classification of their prescribed medications. The Controlled Substances Act was established with the intent of safeguarding public welfare, and adherence to regulatory protocols ensures both therapeutic efficacy and societal integrity. Pharmacists are not merely dispensers-they are custodians of the legal framework that prevents diversion and abuse.
Jason Yan
January 20, 2026 AT 00:02I think the real question isn’t why the schedules exist-it’s why we still treat drugs like they’re either pure evil or pure magic. A pill is just a chemical. It’s the context-your body, your life, your access to therapy, your support system-that turns it into a lifeline or a trap. The DEA doesn’t care about any of that. They care about paperwork. And that’s why people die. Not because they’re addicts. Because the system won’t let them get help without jumping through 17 hoops made of bureaucracy and shame.
shiv singh
January 20, 2026 AT 21:26you think this is bad wait till they start tracking your coffee intake next. next thing you know theyll say your 3pm latte is a schedule iv stimulant because it has caffeine and you might get addicted to it. this is how tyrannies start. one pill at a time.
Robert Way
January 21, 2026 AT 18:21i got my xanax scrip yesterday and the phamarcy said they need to call my doc to confirm the dea number but my doc is on vacay for 2 weeks and i need it for my anxiety. why do i have to suffer because some guy in washington thinks i might sell my pills? its not like im gonna sell them to a 14 year old i just want to not cry in the shower
Sarah Triphahn
January 22, 2026 AT 00:23everyone talks about how bad schedule ii is but nobody mentions that the real problem is the people who hoard them. you know who you are. the ones who take 3 oxycodone a day for a sprained ankle and stockpile the rest like they’re prepping for the apocalypse. you’re not a patient. you’re a drug dealer with a prescription. stop pretending you’re the victim.
Vicky Zhang
January 23, 2026 AT 07:27I just want to say to anyone reading this who’s scared or confused-YOU ARE NOT ALONE. I’ve been there. I’ve cried in the pharmacy parking lot because my refill was denied. I’ve stared at that little "CSA SCH II" label like it was a death sentence. But here’s the truth: you deserve care. You deserve dignity. You deserve to be treated like a human, not a statistic. Talk to your pharmacist. Ask questions. Don’t let shame silence you. You’re fighting for your life-and that’s brave.
Allison Deming
January 24, 2026 AT 12:05The notion that marijuana should be rescheduled to Schedule III is a dangerous concession to political expediency rather than scientific rigor. The federal government maintains these classifications based on decades of peer-reviewed research, not trending social media narratives. To lower the barrier for access without addressing the neurocognitive risks associated with chronic use is to prioritize ideology over evidence-and history has shown that such compromises invariably lead to public health crises.
Susie Deer
January 25, 2026 AT 12:43if you cant handle a little paperwork then maybe you shouldnt be taking drugs. america is weak. we let criminals get free healthcare while real citizens have to beg for pain meds. this is why we lost the war on drugs. because we care more about being nice than being safe
TooAfraid ToSay
January 26, 2026 AT 03:36in nigeria we just buy meds off the street and no one cares. you americans act like you’re the only ones who have pain. you think your system is better? i’ve seen people die waiting for a scrip. here we just get it. no forms no dea no drama. maybe your system isn’t protecting you. maybe its just making you suffer longer
says haze
January 27, 2026 AT 01:05The scheduling system is a relic of moralistic governance disguised as pharmacology. It conflates social stigma with biological risk, and the result is a taxonomy that reflects 1970s prejudices more than 21st-century neuroscience. MDMA, psilocybin, even cannabis-these substances have demonstrated therapeutic efficacy in controlled trials, yet remain locked in Schedule I because of political inertia, not pharmacological logic. The real danger isn’t the drugs. It’s the institutional refusal to evolve.
Alvin Bregman
January 28, 2026 AT 00:29i used to think this stuff was overkill until my sister got hooked on vicodin after surgery. the system didn’t stop her. but it did make it harder for her to get help when she was ready. maybe the rules are right but the way we enforce them is broken. we treat addicts like criminals and people who need meds like suspects. maybe we need to flip that. not more locks. more hands.